Vaccination Design Projects
Breaking Tolerance to Carbohydrates Antigens
The immune system protects us from infection and in some cases cancer. Lymphocytes comprise what is known as the adaptive arm of the immune system, and function to recognise and eliminate infectious and other threats. B lymphocytes (B cells) produce antibodies that bind invading pathogens and cancer cells and eliminate them, whereas T lymphocytes (T cells) can assist B (and other immune) cells and kill pathogen-infected and cancer cells.
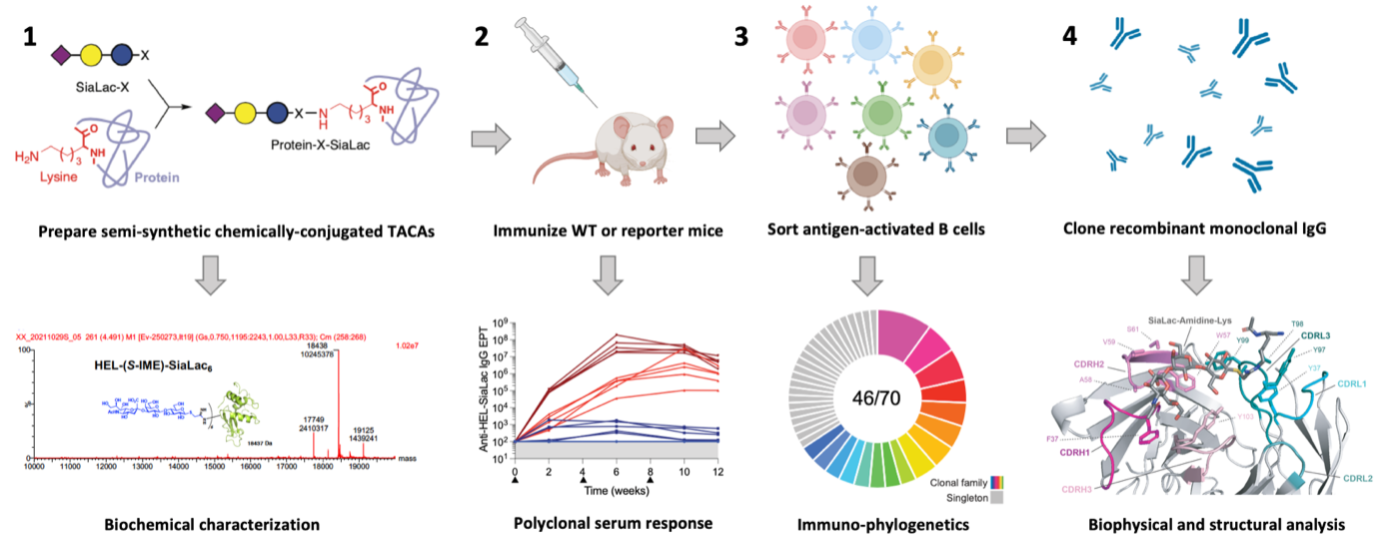
Antibodies are considered to be the major protective immune response elicited by vaccination, and so the study of B cell responses to pathogens and their components is essential to produce the vaccines of the future. This is particularly true of difficult pathogens such as the human immunodeficiency virus type-1 (HIV-1) and cancers against which antibodies may help to protect us. Antibodies conventionally recognise protein targets, but can also engage carbohydrate (glycan) targets on viruses and cancer cells. Since immune escape by pathogens and cancers may be difficult when glycans are targeted, it is of interest to understand how B cells interact with glycans and produce glycan-specific antibodies. We have established systems for isolating single glycan-specific B cells and synthesising and analysing the monoclonal antibodies produced (Figure 1). In this way we are increasing our understanding of antibody-glycan interactions at the cellular, biophysical and structural levels which will guide future anti-pathogen and anti-cancer vaccine efforts.
Checkout our latest preprint here:
Deimel, L. P., Xue, X., Khan, A., Moynie, L., Buchanan, C. J., Sun, G., McBride, R., Schuster, H., Gauthier, C., Saliba, R., Leonavicus, K., Minall, L., Bort, G., Russell, R. A., Sezgin, E., Paulson, J. C., Anthony, D. C., Baldwin, A. J., Naismith, J., … Sattentau, Q. J. (2023). Engineered display of ganglioside-sugars on protein elicits a clonally and structurally constrained B cell response. BioRxiv, 2023.06.03.543556. https://doi.org/10.1101/2023.06.03.543556
Workflow for immunogen design and characterisation, B cell isolation, and clonal analysis.
Immune Responses to Chemically Unstable Drugs
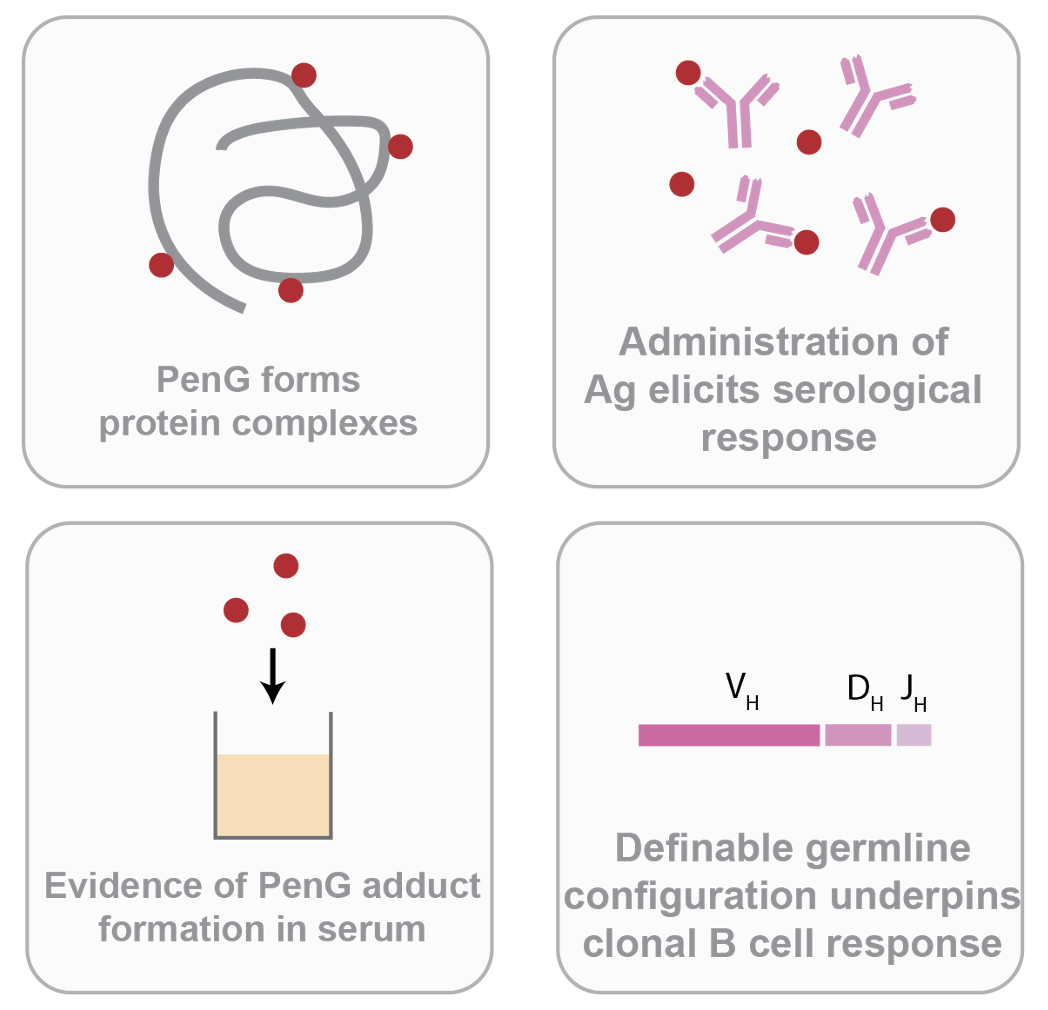
Many small-molecular drugs (SMDs) have constituent chemically reactive functional groups that might form adducts in vivo. These SMDs have long been described as potential “haptens”, where their fixing to protein forms functional antigenic complexes that the immune system can respond to and ultimately produce antibodies against; for example, a B cell can cross-link a hypothetical SMD-encompassing epitope, while associated peptide can be presented to T cells to help and propagate the B cell response.
We have developed a model using penicillin G (PenG), which has long been shown to form adducts that act as the main antigenic determinant for drug hypersensitivity, to evaluate the relationship between drug chemical instability, adduct formation in vivo, and the downstream immunological determinants the facilitate antibody production and allergy.
Characteristics of chemically-unstable drugs and their immunogenicity characterisation.